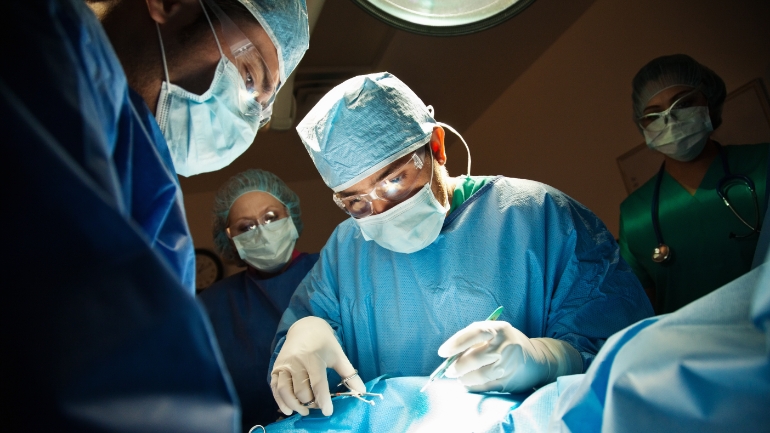
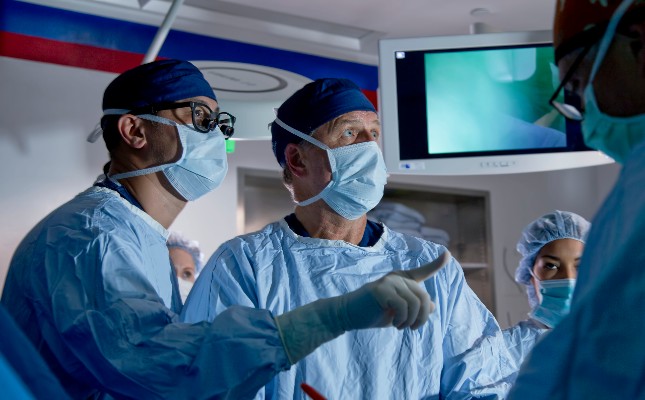
Landmark TOTAL Trial Results Published
After a decade of work, the Tracheal Occlusion To Accelerate Lung Growth (TOTAL) Trial was published. Dr. Anthony Johnson, Co-Director of The Fetal Center* at Children’s Memorial Hermann Hospital and a co-author of the trial, discusses the team’s approach to prenatal intervention and care for patients with Congenital Diaphragmatic Hernia.

Landmark TOTAL Trial Results Published
Caitlin Whyte (Host): Advancing health, personalizing care at Memorial Hermann, this is our mission. This podcast shares the science and stories behind those efforts. Today we're interviewing Dr. Anthony Johnson, who is Co-Director of The Fetal Center at McGovern Medical School at UTHealth Houston and Children’s Memorial Hermann Hospital, to discuss the recently published results of the TOTAL trial, a landmark trial to advance care for patients with congenital diaphragmatic hernia (CDH).
This is Advance, the podcast series from Memorial Hermann. I'm Caitlin Whyte. As we know, Doctor, a landmark research trial focused on patients with congenital diaphragmatic hernia or CDH was recently published in the New England Journal of Medicine. Can you tell us a little bit about this trial and about the purpose of the trial?
Dr. Johnson: Sure, happy to. The trial is called the Tracheal Occlusion To Accelerate Lung Growth. Or the TOTAL trial. It was an international multicenter, randomized trial with two arms, the severe arm and the moderate arm. And, it was basically put into play to evaluate survival and morbidity in fetuses with a congenital diaphragm hernia, or CDH.
The difference between the two arms, the severe arm was based on lung volume, if you will. All right, the severe arm would have less lung volume available and those patients underwent fetal intervention, or randomized to fetal intervention, at less than 30 weeks or roughly less than 7 months. And the moderate arm was looking at not just survival but also long-term morbidity in the neonates and children.
And that's the randomization there resulted in the intervention between 30 and 32 weeks or seven to seven and a half months. The purpose of the trial was really trying to advance the care and understanding and improve the outcomes for children with CDH.
Host: And what were the findings of the TOTAL Trial?
Dr. Johnson: The trial, 10 years in completion, found that the severe group actually benefited from fetal intervention when the surgeries were done between 27 and 30 weeks’ gestation. It improves survival with reduced morbidity in those children.
And that improved outcome actually extended not just in the newborn period, but actually up until 6 months of age. That's as far as we followed them right now. So, we're still looking at the long-term outcome. The moderate arm, when the procedures were done at 30 to 32 weeks or 7 to 7-1/2 months, didn't show an improvement. Both studies though showed that the babies that went through intervention had an increased risk for premature delivery, preterm labor, and premature rupture of membranes. So, it's kind of a balancing act between the two.
Host: Now, just how were you and your organization involved in the TOTAL trial?
Dr. Johnson: I been working with Professor Deprest, the principal investigator of the trial for the past 11 years in this endeavor. Part of our undertaking was trying to incorporate other U.S. centers into the trial to participate.
So, my role was not only as a local co-investigator, but, also, I was a coordinator of the 10 sites, and in North America that moved forward to get this endeavor underway. Of the sites, one was in Canada, nine were in the US. We needed to get FDA approval to move forward with this. So, I was paramount in helping that happen.
And then from there, coordinated things forward. Our program here at Children's Memorial Hermann Hospital was the only fetal center to participate in both arms of the TOTAL trial.
Host: Wow. And has your multiple disciplinary team been involved in other research efforts for CDH?
Dr. Johnson: When I joined the team here roughly 10 years ago, it was based on the work that's being done with CDH, a significant part of that. The program here is amazing with regard to the affiliated teams and basic translational and the clinical scientific work in this area, trying to advance what we need to know and what the new therapies might be. The team here, really is amazing. It leads investigative collaboration internally and also with associated sites. In the past two years alone, there's been over 40 peer review publications coming out in CDH. Fortunately, just before the COVID outbreak here, we were able to sponsor the International Congenital Diaphragm Hernia Symposium. It's an educational program to advance our knowledge in this disorder with 11 different countries participating.
We're constantly moving forward to try and striving to learn more about how to help patients in this area with our expertise. Right now, we're the only center, that I'm aware of, that's actually looking at the use of stem cells in a phase one trial for the treatment of CDH.
Host: Can you tell us just how you and your multidisciplinary team care for patients with CDH?
Dr. Johnson: Yeah. You know, anything you do in the fetal world has to have a postnatal team. I mean, we can do a lot of things here, but if we don't have somebody on the other side of the delivery, that's ready to run with this, you're really just kidding yourself. And that's one of the amazing things about the group here. There's over 30 years of experience with CDH in this center. It's a national referral center for CDH. It's a multidisciplinary team that starts just in the prenatal side with the maternal-fetal medicine specialists, neonatologists, pediatric surgeons, pediatric anesthesiologists and other pediatric specialists.
And it's all integrated together between UTHealth Houston and Children's Memorial Hermann Hospital to really provide quality care. And this diagnosis, again, being full nondisclosure as to what we're doing, what the patient's still going to experience moving forward to make it a smooth transition. The beauty about things here, we start before delivery pre-Apgar, if you will, all the way through to adulthood taking care of these people. It's really based on just an understanding of how this is going to move forward. And part of that process has been able to allow us to have a higher than expected risk-stratified survival rate. One of the highest in the world.
Host: Now, will your center be offering the fetal procedure to CDH patients?
Dr. Johnson: Moving forward. Yes, we will. For those patients that qualify. The evidence shows that there's definitely benefit. Okay. And we will continue to advance this looking at different modifications in this. We're going to continue to collaborate with the centers that have been working with this to move forward.
We're working with manufacturing and the FDA to see how we might be able to move this forward into the US. With a comprehensive program, we feel confident we can continue to provide expertise in this area and sort of tailor the management. We're looking at actually introducing this technology into newer populations that have additional funds that would have been excluded in the original trial.
The team's committed to providing the specialty care to families with CDH. We're all about the patient. Okay. Making it convenient. We know they're anxious when they have these diagnoses. So, we make ourselves (available) pretty much 24/7, not actually to the physician to help get the referral here, but actually to the patient, telling them what to expect when they come in.
Sometimes, you know, they're a far distance away and we receive referrals from some 39 different states. So, it can be virtual. Picking up the phone, happy to call you. This is my cell phone number. Just please let us know if we can help. That's part of what we do because we know they're overwhelmed, and we want to sort of calm it down. We can help them.
Host: Talking more about moving forward, what are the next steps in CDH research?
Dr. Johnson: Well, one of the things is going back and looking at a re-evaluation of the moderate arm. One of the big differences between that and the severe is when the procedure was done and looking at some of the preliminary information that would suggest that this moderate group actually may still show benefit if we back the procedure down. In other words, treating them all at the same time, at that 27 to 29 weeks. There's some other ongoing work, looking at different modifications of the device that we might be able to minimize risk to the mother by changing the procedure, so that she doesn't undergo two procedures when she's having this fetal intervention, and seeing if we can go back and reanalyze the U.S. data to find a way that will sort of standardize things moving forward and helping other centers move forward and allowing patients to have access to this device.
Host: Great. Well, Doctor, where can we find out more information about this trial and your program?
Dr. Johnson: Sure. Online, you can go to the Memorialhermann.org/totaltrial, or you can give us a call 832-325-7288.
Host: Well, thank you Doctor for your time, and for this information today. To learn more or to refer a patient, visit Memorial hermann.org/total trial.
That's Memorial Hermann, H-E-R-M-A-N-N.org, or call 832-325-7288. That's 832-325-7288. If you found this podcast helpful, please share it on your social channels and be sure to check out the entire podcast library for topics of interest to you. This has been Advance, the podcast series from Memorial Hermann. I'm Caitlyn Whyte. Stay well.
Located within the Texas Medical Center, The Fetal Center is affiliated with McGovern Medical School at UTHealth Houston, UT Physicians and Children’s Memorial Hermann Hospital.
Companion Article
A Deeper Dive: Beyond the Landmark TOTAL Trial
An estimated 1 in every 3,600 babies in the United States is born with congenital diaphragmatic…